Overview
We are a group of computational physical organic chemists at the University of Houston. We enjoy our "screen-time." But even more so the chemical insights we get out of it.
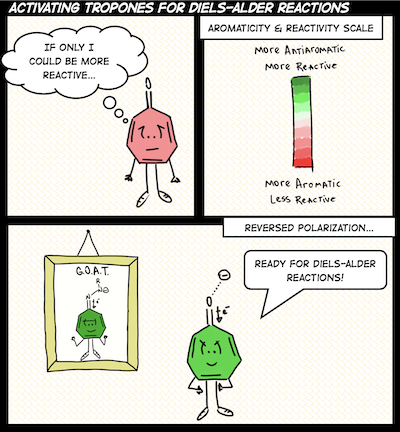
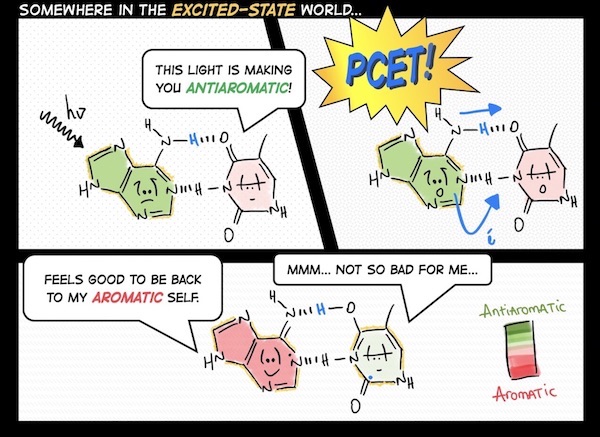
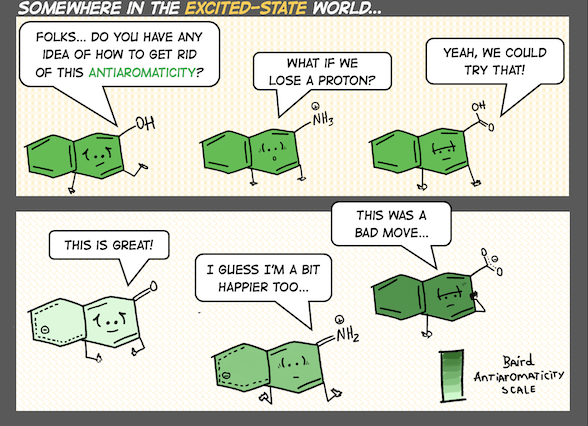
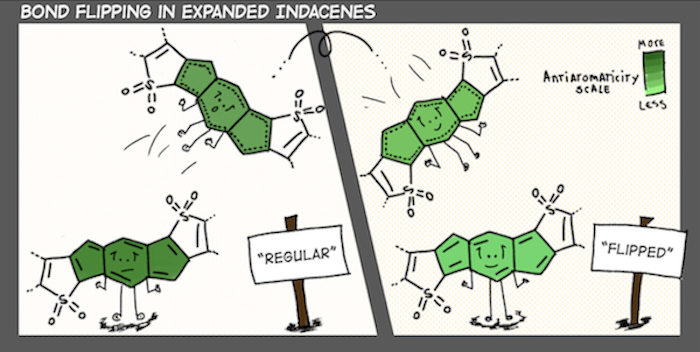
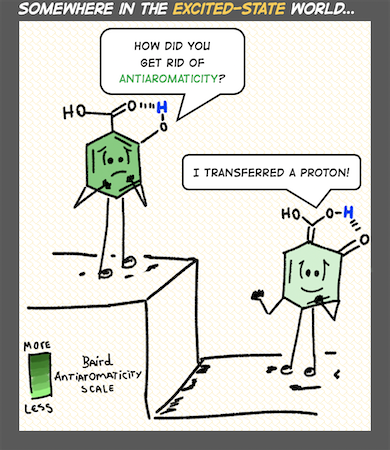
A central theme of our work is recognizing the interpretive power of fuzzy chemical concepts (like aromaticity) in chemistry, and developing useful chemical strategies with them. We are sometimes organic chemists, sometimes photochemists, and other times supramolecular chemists. Right now, topics that catch our attention include light-responsive hydrogen bonds, organic photochemistry and proton-coupled electron transfer reactions, ground and excited-state reactivities and properties of polycyclic (anti)aromatic π-systems.